Vega Stem Cell, a leading regenerative medicine provider operating under Vega Medical Services, is proud to announce a key advancement in its ongoing pursuit of excellence in stem cell science. Through a formal partnership with the Thailand Institute of Scientific and Technological Research (TISTR), Vega has engaged the Department of Medical Sciences (DMSC), under the Ministry of Public Health, to carry out multilineage differentiation testing on its proprietary mesenchymal stem cell (MSC) lines. This testing process is crucial for confirming the identity, potency, and therapeutic viability of stem cells.
This milestone further reinforces Vega’s commitment to internationally accepted quality assurance (QA) and quality control (QC) practices, positioning the company as a leader in safe, science-backed cellular therapy in Thailand and across Southeast Asia.
As stem cell-based treatments gain global momentum for managing chronic diseases, immune conditions, and tissue repair, robust verification protocols are essential to ensure not only clinical effectiveness but also compliance with regulatory and ethical standards.
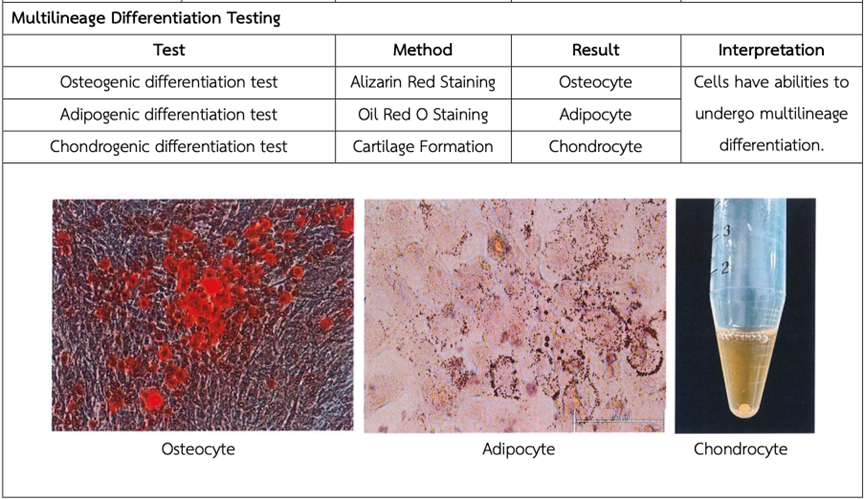
At the core of this verification is multilineage differentiation testing—a benchmark for confirming a stem cell’s true multipotency. In the case of MSCs, this involves their capacity to differentiate into three primary cell types:
- Adipocytes – representing fat tissue, key in metabolic and soft tissue regeneration
- Osteocytes – essential for bone formation and skeletal repair
- Chondrocytes – responsible for cartilage production, relevant in joint restoration and arthritis treatment
These differentiation pathways form the basis for many clinical applications, including regenerative procedures for osteoarthritis, bone trauma, and soft tissue defects. By demonstrating successful in vitro differentiation into these lineages, Vega confirms that its MSCs are biologically functional and therapeutically relevant.
The DMSC, Thailand’s foremost authority in medical testing and regulatory oversight, will conduct the following procedures:
- In vitro induction assays using lineage-specific biochemical media
- Staining and imaging to identify hallmark features such as lipid droplets (for adipocytes), calcium deposition (for osteocytes), and extracellular matrix synthesis (for chondrocytes)
- Molecular analysis to confirm gene expression profiles aligned with each differentiated cell type
This independent, third-party testing by DMSC provides validated scientific evidence that Vega’s stem cells are functionally competent—not only meeting phenotypic markers of MSCs but also exhibiting the regenerative behaviour required for therapeutic application.
Vega’s collaboration with TISTR ensures that the process remains scientifically rigorous, locally integrated, and globally aligned. As a nationally recognized research institution, TISTR offers a robust framework for regulatory preparation, laboratory support, and technology translation—accelerating the deployment of safe and effective regenerative therapies in clinical practice.
In addition to routine sterility and viability assessments, Vega’s decision to include multilineage differentiation testing in its QA/QC protocol highlights its dedication to advancing credible and evidence-based stem cell treatments. Vega’s full QA pipeline includes:
- Careful donor screening for pathogen-free, healthy biological material
- Controlled cell expansion in ISO-classified cleanrooms
- Detailed batch records ensuring traceability and accountability
- Functional validation through differentiation and potency assays
This level of diligence ensures that Vega’s stem cells are not only safe but also clinically robust and prepared for future clinical trials and international collaborations.
Furthermore, this testing initiative aligns Vega with globally recognized standards, such as:
- ISCT guidelines for defining and characterizing MSCs
- Good Manufacturing Practice (GMP) principles for therapeutic cell production
- Regulatory frameworks established by the FDA (USA), EMA (Europe), and other major health authorities
By meeting these benchmarks, Vega positions itself as a serious global contender in the field of regenerative medicine—delivering scientifically validated, ethically sourced, and clinically ready stem cell products.
As unproven and unregulated stem cell offerings continue to emerge worldwide, Vega stands apart by committing to transparency, data-driven validation, and patient-centered safety. The integration of multilineage differentiation testing into its standard procedures exemplifies the kind of rigorous development that inspires trust among patients, clinicians, and regulators alike.
Through its partnerships with DMSC and TISTR, Vega Stem Cell is setting a new precedent for responsible biotechnology advancement in Thailand. With verifiable science at its core, the company continues to champion the safe evolution of regenerative medicine—ensuring that stem cell therapies are not just promising, but also proven, repeatable, and globally respected.
Contact Information
Vega Medical Services
3/6, The Primary 101, Soi Lad Phrao 101
Lad Phrao Road, Khlong Chan
Bang Kapi District, Bangkok 10240
Thailand
Email: vegastemcell@gmail.com
Tel & WhatsApp: +66 (0) 86 691 6915
Website: www.vegastemcell.com

