Vega Stem Cell (vegastemcell.com), a prominent leader in regenerative medicine and stem cell education across Southeast Asia, has successfully cultured high-quality human mesenchymal stem cells (MSCs) using the same culture media and protocols as AGEM Bio, a biotechnology company originating from the National University of Singapore (NUS). This accomplishment verifies that Vega’s cell culture methods meet internationally accepted standards, including those set by the Pharmaceutical Inspection Co-operation Scheme (PIC/S) Good Manufacturing Practices (GMP) enforced in Singapore.
This achievement underscores Vega Stem Cell’s strong dedication to global collaboration, strict scientific validation, and commitment to advancing stem cell therapies and education. The ability to replicate AGEM Bio’s results at Vega’s facility marks a crucial milestone in unifying stem cell technology standards across countries.
A New Benchmark in Stem Cell Production
Stem cells are at the forefront of regenerative medicine due to their ability to differentiate into various cell types and promote tissue repair. High-quality cell culturing protocols are essential to ensure the consistency, safety, and therapeutic efficacy of stem cell treatments. Working closely with AGEM Bio, Vega Stem Cell adopted the STEMGOLD™ protocol—an advanced, xeno-free culture medium specifically optimized for human MSC expansion without compromising cell viability or potency.
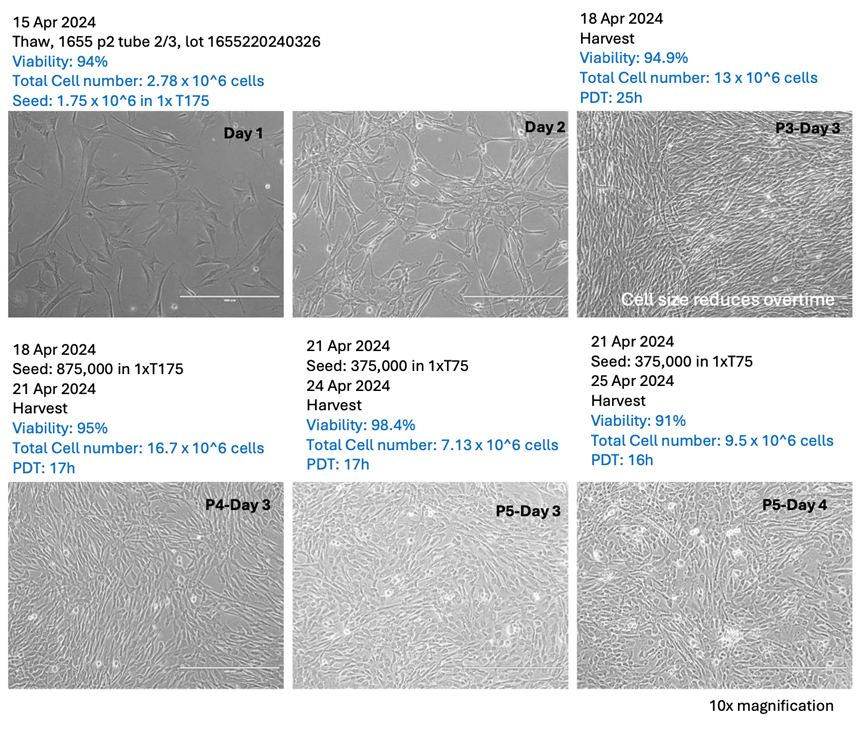
Over a nine-day production cycle using STEMGOLD™ media, Vega was able to expand MSCs from a single P2 vial to a total of 7.5 billion cells by passage five (P5), while maintaining viability rates consistently above 90%. These results mirror those achieved by AGEM Bio, confirming that Vega’s internal processes meet international GMP-level benchmarks.
Collaboration with International Partners
Vega Stem Cell’s cooperation with AGEM Bio demonstrates the importance of international partnerships in advancing cell therapy development. AGEM Bio is a recognized innovator in stem cell media technology.
Through this collaboration, the parties collectively evaluated the scalability, consistency, and clinical-grade viability of MSC production using STEMGOLD™ media. The results not only reaffirmed the quality of AGEM’s proprietary media but also validated Vega’s capacity to implement and sustain best-in-class bioprocessing methodologies.
Such partnerships are vital to the global integration of stem cell science, enabling the sharing of protocols, quality standards, and clinical insights across borders. These efforts are paving the way for the adoption of unified regulatory frameworks, particularly in emerging markets such as Southeast Asia.
Stem Cell Treatments and Future Applications
The ability to produce high-quality stem cells at scale unlocks a broad range of therapeutic applications. MSCs are being actively investigated and applied in the treatment of:
– Orthopedic injuries and degenerative joint conditions
– Chronic wounds and diabetic ulcers
– Immune-related diseases and inflammation
– Neurological and cardiovascular disorders
– Dental tissue regeneration, including bone grafting and periodontal repair
By aligning its practices with GMP-compliant standards, Vega Stem Cell ensures that its therapies are ready for clinical deployment and scalable production. The company is currently exploring multiple pipelines, including cell-based treatments in dentistry, dermatology, and musculoskeletal care.
Commitment to Stem Cell Education and Training
In addition to clinical innovation, Vega Stem Cell is deeply committed to education. Recognizing the need for well-trained medical professionals, Vega is establishing itself as a regional leader in stem cell education and training. The company offers academic and hands-on training programs that empower practitioners to understand and apply cell therapy safely and effectively.
These programs cover a wide array of critical subjects, including:
– Stem cell biology and classification
– GMP standards for stem cell processing
– Cell handling and cryopreservation
– Clinical application techniques in orthopedics, aesthetics, and dentistry
– Regulatory and ethical considerations in regenerative medicine
By integrating real-world case studies and validated lab protocols, Vega ensures that participants not only gain theoretical knowledge but also the clinical skills necessary to practice in a rapidly evolving medical landscape.
Education is not an auxiliary function—it is a core pillar of Vega’s mission to elevate regional expertise and make regenerative medicine more accessible across Asia and beyond.
Upholding Global Standards for Quality and Safety
One of the cornerstones of Vega’s operations is its unwavering commitment to international quality standards. As regenerative medicine continues to expand, ensuring consistency, traceability, and safety in stem cell treatments is paramount.
The successful replication of AGEM Bio’s results using STEMGOLD™ media underscores Vega’s ability to meet PIC/S GMP criteria. This is crucial for institutions and healthcare systems that prioritize high-quality biological products that are safe for patient use.
Furthermore, adherence to such standards enhances Vega’s readiness to participate in international clinical trials, support regulatory submissions, and expand its services across borders.
A Forward-Looking Strategy for Scalable Cell Therapy
This collaborative study serves as a blueprint for future partnerships that prioritize excellence, education, and international compatibility. As stem cell treatments become more prevalent, the ability to produce high-quality cells consistently and ethically will define the credibility and success of providers.
Vega Stem Cell continues to position itself at the forefront of this transformation—investing in training, expanding its lab capabilities, and aligning with global standards to ensure the long-term impact of its therapies.
Contact Information
Vega Medical Services
3/6, The Primary 101, Soi Lad Phrao 101
Lad Phrao Road, Khlong Chan
Bang Kapi District, Bangkok 10240
Thailand
Email: vegastemcell@gmail.com
Tel & WhatsApp: +66 (0) 86 691 6915
Website: www.vegastemcell.com

