Vega Stem Cell (vegastemcell.com), a trailblazer in regenerative medicine and stem cell education, has achieved a major advancement through its collaboration with AGEM Bio, a pioneering biotechnology spin-off from the National University of Singapore (NUS). AGEM Bio has successfully validated the compatibility of Vega’s cultured stem cells with its proprietary non-viral gene delivery system, confirming that these cells meet rigorous international criteria suitable for cancer-focused therapeutic applications.
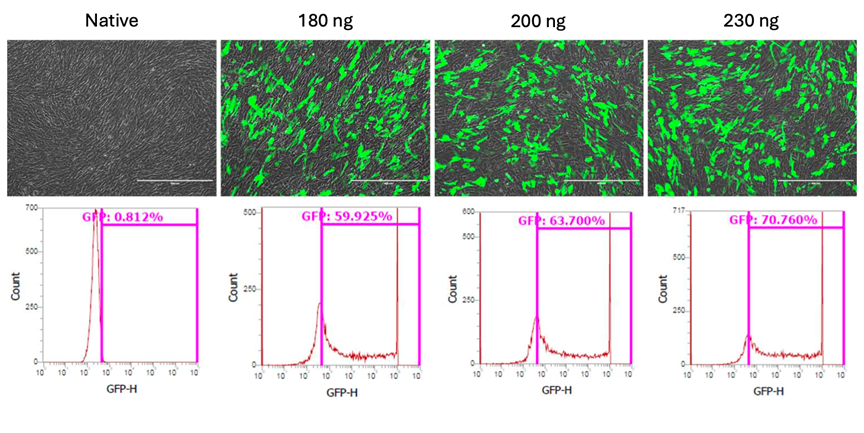
In a landmark experiment conducted on April 25, 2024, AGEM Bio utilized its sophisticated transfection technique on mesenchymal stem cells supplied by Vega Stem Cell. The cells were engineered to express Green Fluorescent Protein (GFP) with transfection efficiencies rising in accordance with plasmid concentration, peaking at 70.76% expression at 230 ng. This outcome validates the robustness and adaptability of Vega’s stem cell culturing methods within globally recognized frameworks for cutting-edge cell therapies, including those aimed at oncology.
The Power of Stem Cells in Oncology and Regenerative Medicine
Stem cell treatments are revolutionizing medicine, especially in areas such as regenerative repair, immunomodulation, and now, cancer therapy. Mesenchymal stem cells (MSCs) possess intrinsic tumor-homing capabilities that make them ideal vectors for delivering anti-cancer payloads. When engineered correctly, these cells can infiltrate tumor environments and deliver therapeutic agents directly to malignant tissues.
The successful modification of Vega-cultured MSCs using AGEM Bio’s non-viral gene transfer method demonstrates that these cells can serve as a viable platform for precision oncology applications. Importantly, this transformation was achieved without using viral vectors, thereby reducing the risk of insertional mutagenesis and enhancing the safety profile of the therapy—critical considerations for clinical translation.
Validation Through International Collaboration
This achievement is the result of strategic collaboration between two institutions dedicated to pushing the boundaries of regenerative medicine: Vega Stem Cell in Thailand and AGEM Bio in Singapore. The ability of AGEM Bio to replicate its results using Vega’s MSCs shows a clear convergence in quality control, culturing technique, and biological viability.
This kind of cross-border validation is crucial in today’s biomedical landscape. International collaborations enable biotechnology stakeholders to synchronize protocols, reduce barriers to clinical trials, and accelerate therapeutic development. Vega’s compatibility with AGEM Bio’s standards affirms that Thailand-based facilities can meet or exceed the benchmarks of global biotech leaders.
Such partnerships ensure not just reproducibility, but also scalability—an essential feature for translating stem cell research into real-world treatments.
Vega’s Commitment to Global-Standard Stem Cell Training and Education
Beyond therapeutic applications, Vega Stem Cell remains committed to advancing stem cell education and workforce training in Southeast Asia. As regenerative medicine moves from research labs into hospitals and clinics, the demand for knowledgeable, technically skilled practitioners continues to rise.
Vega’s educational programs are designed to produce world-class professionals equipped with both theoretical understanding and practical skills in stem cell biology, processing, and clinical application. These programs include:
– Fundamentals of stem cell science
– GMP-compliant culturing and handling
– Cryopreservation and expansion techniques
– Clinical deployment in dermatology, orthopedics, and dental regeneration
– Regulatory ethics and international compliance
By aligning its curriculum with global standards, Vega ensures that graduates can contribute meaningfully to international biomedical projects and trials.
High-Performance Media for Consistent Results
The stem cells used in the collaboration with AGEM Bio were cultured in STEMGOLD™, a xeno-free, high-quality stem cell medium known for its consistent ability to enhance mesenchymal stem cell (MSC) yield and viability. Over a nine-day culture period, Vega successfully expanded MSCs from a single vial to more than 7.5 billion cells by the fifth passage, maintaining cell viability above 90% throughout. These outcomes closely match those achieved by AGEM Bio in Singapore, demonstrating Vega’s strong technical expertise and capacity.
Beyond its scalability, STEMGOLD™ offers a highly reproducible culture environment, ensuring that key stem cell properties—such as cell shape, transfection efficiency, and growth rate—remain stable between laboratories and production batches. This consistency is vital in clinical applications, where variability can compromise both the safety and effectiveness of treatments.
Next Steps in Precision Oncology and Beyond
The successful validation of Vega Stem Cell’s cultured mesenchymal stem cells (MSCs) within AGEM Bio’s gene therapy program for solid tumors marks a significant advancement in targeted cancer treatment. This achievement paves the way for future projects, including:
- Broader research on stem cell-based immunotherapies
- Clinical trials focused on treating solid tumors with genetically modified MSCs
- Creation of stem cell-driven drug delivery platforms
- Regulatory harmonization across multiple countries to facilitate approval and adoption
As this area of medicine advances, partnerships such as the one between Vega Stem Cell and AGEM Bio are essential to ensure that new discoveries are quickly translated from the laboratory into treatments that benefit patients worldwide.
Thailand’s Role as a Biomedical Innovation Hub
Thailand is rapidly establishing itself as a leading center for biomedical research and healthcare innovation. With institutions like Vega Stem Cell driving high-standard cell therapy programs and forming international alliances, the country is poised to contribute significantly to the global regenerative medicine ecosystem.
This latest success story between Vega and AGEM Bio is a strong indication that Thailand can not only support high-end research but also produce cell therapy products that are ready for clinical application and international validation.
Maintaining the Highest Standards
As stem cell therapies inch closer to mainstream adoption, maintaining global manufacturing and safety standards becomes more important than ever. Vega’s ability to meet the standards set by its Singaporean counterpart underscores its readiness to participate in international clinical development, product licensing, and research collaborations.
With a focus on reproducibility, scalability, and patient safety, Vega continues to invest in advanced infrastructure and international benchmarking. These efforts are supported by strict internal QA/QC protocols, professional training programs, and compliance with PIC/S GMP guidelines.
Contact Information
Vega Medical Services
3/6, The Primary 101, Soi Lad Phrao 101
Lad Phrao Road, Khlong Chan
Bang Kapi District, Bangkok 10240
Thailand
Email: vegastemcell@gmail.com
Tel & WhatsApp: +66 (0) 86 691 6915
Website: www.vegastemcell.com

